Hearing Aid Batteries: The Past, Present, and Future
Heike Heuermann, PhD, Rebecca Herbig, AuD
AudiologyOnline, October 19, 2016
Introduction
Modern digital hearing aids offer a huge variety of form factors, features, and wireless connectivity options that allow for individual hearing solutions. However, the price of functions like situation-based real-time processing, binaural algorithms, or streaming is an increased demand on battery performance. So far, the topic of efficient powering has received only scant attention, but this may change soon due to the many good reasons for using rechargeable batteries.
Battery Basics
Why is it important to think about hearing aid batteries? Batteries are the most practical solution when it comes to powering smaller electronic devices of every kind. Rechargeable batteries have found their way into our daily life for almost every device, yet a huge number of disposable batteries are still being used. An estimated 1.4 billion disposable hearing aid batteries are dumped in landfills annually (Dueber, 2014). On the other hand, there is an ongoing trend toward greater environmental awareness and “green” technologies. Maybe consumers are just lacking the right solution?
Hearing aid users have been expressing a high interest in hearing aid batteries and their ease of use for a long time. In the MarkeTrack VI survey (Kochkin, 2002), about 80 percent of US consumers requested batteries that last longer, and about half of the users requested hearing instruments featuring easier-to-change batteries. The only other features that received an equally high ranking were hearing aid volume control, remote control, and the like.
The picture has not changed much since then. In the most recent MarkeTrak 9 (Abrams & Kihm, 2015), potential new hearing aid users were asked what they consider to be the most compelling features. A rechargeable hearing aid and/or rechargeable batteries for hearing aids were rated as more important than any digital feature or wireless connectivity.
This is not surprising, since rechargeable batteries offer a number of advantages, including:
- Greater comfort and ease of use: No need to deal with small batteries on a regular basis. Depending on the solution, hearing aids could simply be placed into a charger at night. This is particularly beneficial for users with dexterity challenges.
- Environmentally friendly: Over a three-year time span, two digital hearing aids will use an average of more than 300 disposable hearing aid batteries. An average of only two lithium-ion rechargeable batteries would be used under the same conditions.
- Cost effective: A single zinc-air battery costs an average of $1. Assuming the typical bilateral hearing aid wearer changes both batteries once a week, costs can exceed $300 during a three year period.
- Convenience: No need to remember to purchase new batteries or locate them when they are needed.
- Peace of mind: Knowing that the hearing aid batteries won’t go dead in the middle of the day, perhaps during an important meeting or event.
Functional Principles of Batteries
Every classical and state-of-the-art battery generally follows the same principle (see Figure 1). It typically consists of at least four components:
- The anode, or negative electrode, gives up electrons to the external circuit. It is generally a metal, which is oxidized during discharge.
- The cathode, or positive electrode, accepts electrons when the battery is connected to an electric circuit. It is usually a metallic oxide or a sulfide, but oxygen is also used (e.g., in zinc-air batteries).
- The electrolyte provides the medium for the transfer of ions inside the cell between the anode and cathode. During battery discharge metal atoms that are missing electrons move through the electrolyte towards the cathode.
- Often a separator is required to electrically isolate the positive and negative electrodes.
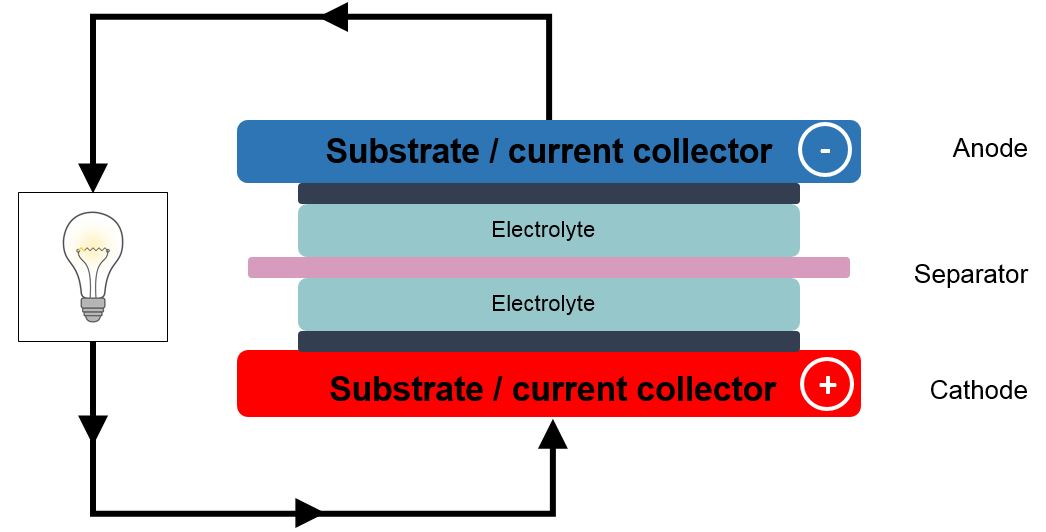
Figure 1. Basic components of a battery. Every battery consists of an anode (electron donator), a cathode (electron receptor or reducer), and an electrolyte enabling the conduction of metal ions. Electrons cannot pass through the electrolyte, so they are forced to travel through the electric circuit during the chemical process of metal oxidation. Separators are sometimes needed to physically isolate cathode and anode, and prevent short-circuits.
When the battery is fully charged, a surplus of electrons on the anode gives it a negative charge and a deficit on the cathode a positive charge. This results in an electric potential difference, or voltage, across the cell.
When the circuit is closed, the surplus electrons flow into the external circuit from the anode, which loses all its charge, to the cathode, which accepts it, neutralizing its positive charge. This action reduces the potential difference across the cell to zero. The circuit is completed or balanced by positive ions dissolving from the anode into the electrolyte and flowing to the cathode.
What determines the performance or capacity of the battery is the type of anode and cathode materials, e.g., zinc-copper, zinc-air, or nickel-metal hydride. We can generally distinguish between primary cells (non-rechargeable) and secondary cells (rechargeable). Secondary cells are able to reverse the electro-chemical process of discharge.
Zinc-air Batteries
This is the most commonly used hearing aid battery. It is activated by removing the sealing tab allowing oxygen from the air to pass into the cell and start a two-step chemical process. This chemical reaction produces about 1.35–1.4 V in the standard battery types.
Zinc-air cells come with a number of advantages:
- Relatively high energy density
- Inexpensive materials
- Constant voltage rate over a relatively long time
- Very low self-discharge rate under sealed condition
- Relatively long-lasting under low power conditions
- Proven technology
Yet they also have some shortcomings, which should keep us looking for alternative solutions. For instance, once the seal is broken self-discharge starts irrevocably and at a high rate. They are sensitive to extreme temperatures and humidity. Finally, they are the typical representative of primary cells (i.e., not rechargeable).
Nickel Metal Hydride
Nickel metal hydride (NiMH) cells are a long-proven type of rechargeable (secondary) battery. The automobile industry triggered their initial development, and many of us may still remember cameras being powered by NiMH batteries. Its chemical process is not based on external oxygen, which means that the cells remain sealed during normal operation and are thus maintenance-free.
NiMH cells share some advantages with zinc-air batteries: they come with a comparable energy density and show a constant voltage rate over a long time. They are available in all standard hearing aid battery types, and their voltage is comparable to zinc-air cells. These capabilities made NiMH a trusted and preferred solution for rechargeable hearing aids for a long time, because both NiMH and zinc-air types can be used (and interchanged whenever desired) for the same instrument.
However, there are also some challenges related to NiMH. First of all, they exhibit a relatively high self-discharge rate, especially at higher temperatures. Second, they can be overcharged easily, which dramatically reduces their overall lifetime. Ultra-low discharge or pole-reversal can also seriously damage the cell, leading to sudden battery death ― one reason that may have led to their quick disappearance in the camera industry once newer technologies came to market. The risk of overcharging or pole reversal can be reduced by using “intelligent” charger solutions. These have been available and successful on the market for a couple of years now. But the biggest disadvantage remains ― the energy density might be good compared to zinc-air, but like zinc-air it will not be sufficient to satisfy the needs of modern, binaurally-streaming hearing aid system users.
Silver-zinc
One prominent and extensively discussed example of secondary cells in hearing aids is the silver-zinc (AgZn) battery. Silver-zinc batteries have been used for years in military and aerospace applications. In the early 2000s, a California-based company started developing AgZn batteries for notebooks as an alternative source for lithium-ion batteries. They decided to move into the smaller-sized hearing aid battery business in 2009.
The basic structure of an AgZn cell is similar to a conventional zinc-air battery. It consists of a zinc anode and a silver oxide (AgO) cathode. Electrons are transported through an external circuit from anode to cathode, providing power at about 1.85V.
AgZn cells have a higher energy density than zinc-air batteries and NiMH batteries while maintaining good discharge rate capability. They are non-flammable and fully recyclable. Since they come with the same form factors as traditional zinc-air batteries, it is sometimes assumed that they are interchangeable (see Cui (2014) for an example). However, this is not the case, and the wrong use of AgZn will seriously damage the hearing aid. The reason for this is the original voltage of 1.85V still needs down conversion to 1.3V. Otherwise, the instrument’s battery contacts and nearby electronics will quickly corrode and eventually destroy it. Hearing aids meant to be used with AgZn batteries need a down converter to be mechanically added (e.g., in the battery door), which increases the size of the hearing aid and compromises its cosmetic appeal. The fact that it is not interchangeable with regular, disposable batteries also means that the end user needs to purchase and carry around two additional, relatively expensive AgZn spare batteries as a back-up.
An additional disadvantage of silver-zinc cells is the comparatively short life cycle (current estimations are about one year) in combination with the high price of a new cell.
Lithium Ion
Lithium-based batteries as such are not really new. Primary (non-rechargeable) lithium-ion batteries have been around since the 1960s, first in military and aerospace technology, then later in medical devices and consumer electronics. Because of their ultra-low self-discharge rate, they are still used in pacemakers and watches.
The triumphal course of secondary (rechargeable) lithium-ion batteries started in the 1980s with their wide-spread use in mobile phones, cameras, laptops, and even cars.
Lithium-ion cells are different from the traditional battery types ― they define a completely new family of rechargeable cells. More precisely, there is no one specific lithium-ion battery. Rather, multiple chemistries are possible. The most prominently used materials are LiCoO2, LiFePO4, LiMnO4, Li2TiO3, or LiMnCoO2 and the anode is typically a carbon lattice. The name “lithium-ion battery” explicitly describes what happens in this cell type ― it is these very ions that keep the battery running. During charging, the cathode gives up the lithium-ions, which move to the carbon host lattice. During discharge, the lithium-ions move back, setting free the energy that had been used to make them move to the lattice. Cell voltage depends on the cathode material, but mostly it can be considered to be around 3.8V.
Although the working principle sounds very simple and similar to that of other battery types, lithium-ion batteries are technically much more advanced. Due to the more complex chemistry and physical setup of the cells, electronic controllers are required to regulate the process of charge and discharge.
Lithium-ion (Li-Ion) batteries come with the following advantages, which explains their widespread use in all kinds of consumer electronics.
Very high energy density and energy efficiency. They can run an electronic device for a long time even under high demands. We all know the battery life in a hearing aid has decreased in past years because of more demanding features, such as streaming and binaural functions. Lithium-ion cells can be the solution.
The significantly longer battery life brings significant benefits in terms of usability. With a runtime of up to 24 hours, even with wireless streaming and binaural features engaged on a single charge, wearers no longer have to worry about the hearing aid “running out of battery” in the middle of the day. It also eliminates the need to purchase and carry around spare batteries for such occurrences.
Fast charging mode. Li-Ion technology allows for a fast charging mode, so when provided with good charging technology, a hearing aid can be used up to another seven hours after only 30 minutes of charging. In the unlikely case that a hearing instrument needs to be recharged during the day (e.g., because the user forgot to charge it overnight), this fast-charging mode is another convenience.
User-friendly charging capabilities. Li-Ion batteries can be charged very rapidly with high capacities. In contrast to many previous types of rechargeable batteries, they don’t show memory effects.
Long lifecycle and very low self-discharge rate. A long lifecycle and a very low self-discharge rate means a long life on the shelf as well as in the wearers’ instruments.
Easy to handle. Like with NiMH batteries, Li-Ion cells are sealed and maintenance-free, which makes them easy to handle. They can be kept in the hearing instrument their entire lifetime ― a relief for individuals with dexterity or vision limitations. Remaining sealed inside the hearing aid also means that the battery is less likely to be accidentally swallowed by small children or pets. Even if the entire hearing aid is swallowed, it remains well-protected within the hearing aid housing, preventing it from coming into contact with internal tissue.
Multiple sizes/form factors. Li-Ion technology allows for multiple sizes and form factors, as can be seen from the large variety of Li-Ion batteries in smartphones, cameras, laptops, and cars.
Potential for high energy density. Li-Ion as a whole family of cell types shows much more potential for higher energy density since new chemistries are still being developed. It is therefore a comparatively future-proof technology.
Therefore, Li-Ion batteries seem to be the perfect solution for hearing aids, but if so, why haven’t they been introduced for use with this technology? The reason lies within its complex chemistry, which makes the development of very small batteries a significant challenge ― but one that has finally been overcome.
Under stress conditions like extreme pressure or heat, Li-Ion batteries show a tendency to overheat. Some of us may have already observed that mobile phones get warm under heavy use, which is also a mild symptom of battery stress. We should always select original parts when we replace our smartphone battery in order to maintain the well-proven chemistry inside (remember that there are multiple chemistries possible). Similar approaches hold true for hearing aid batteries ― only long-proven battery manufacturers can be regarded as suitable for the production of small lithium-ion cells required for hearing aids. These manufacturers routinely perform internal stress tests to confirm that neither explosions nor flames can occur under typical conditions.
Other ways of imposing stress on rechargeable batteries include over-charging or ultra-low discharge and even the best chemistry inside could be harmed if not maintained correctly. That means elaborate battery management circuits are required to prevent over- or under-charging. This includes charging technology as well as battery management in the electronic device itself, which we will discuss in the next section. Here we can build on the many years of charging technology experience in the area of NiMH.
Altogether, Li-Ion batteries can be regarded as the technology of choice used in almost all areas of professional and consumer electronics. Their recent introduction to hearing aid application (Kraemer & Naumann, 2016) establishes another milestone in both battery and hearing aid technology. Their user-friendliness sets new standards and their energy efficiency is the basis for sophisticated algorithms and modern streaming applications.
Charging Technologies
Normal vs. Fast Charging
Charging technologies can generally be split into two categories: those that slowly recharge with constant low current and those that use high current for (ultra-) fast charging. As mentioned earlier, over-charging must be avoided regardless of battery type.. For low-current charging, this can be easily achieved by converting surplus charge current into heat. In case of low current, this means a moderate increase of temperature, e.g., warm to the touch. This can be regarded as acceptable for batteries. The downside of this method it that charging can be a lengthy process of up to 10 hours.
If faster charging is desired, higher currents can be used, but then the chargers need to be able to detect when a battery is almost full. Otherwise, the excess heat converted from the higher amount of surplus charge can severely damage or even destroy the cell.
The challenge is how to accurately monitor the charging status. Modern “intelligent” chargers check the battery status by ongoing measurement of temperature and current. If a battery is almost fully charged, the charge current starts to produce heat, and the increasing temperature leads to a mild decrease of battery current. Intelligent chargers are capable of sensing this mild decrease, and recognize that the battery is full. In addition, the charging process is typically stopped if the overall temperature of a battery cell exceeds 45 to 50°C (based on manufacturer’s specification).
Li-Ion batteries always require special charge controllers due to their more delicate chemical setup. Dedicated electronic circuits are required to control the charging current, and even more importantly, the current voltage. Both need to be constantly monitored and limited to avoid over-charging, and therefore over-heating.
Galvanic vs. Inductive Charging
Most conventional battery chargers are still based on galvanic charging ― electronic contacts on a cradle physically touch their counterparts on the electronic device so that the device can be charged. This comes with a number of potential issues. First, devices always need to be placed at an exact position in the cradle so that the electric contacts are well-aligned. Again, this can be a burden for those with vision or dexterity limitations. Additionally, electrodes on the charger’s cradle are always powered when the charging device is put in, which leads to additional charger energy consumption. Although this might be regarded as negligible, it is not in line with the idea of “green energy” usually associated with rechargeable products. Finally, some countries (e.g., Japan) do not allow electronic connections on hearing aids or any other medical device. This means that galvanic charging solutions could not be offered to users in these countries.
A potential solution to these problems is offered by wireless, or inductive, charging that eliminates cables, connectors, and electrodes on the instruments. Maybe the best known application of this technology is high-end smartphones.
Inductive charging works via electro-magnetic induction. In the charger, alternating current (AC) causes a varying magnetic field in a dedicated transmitter coil. This magnetic field in turn induces a similarly varying magnetic field in the receiving coil of the device when the device is in close proximity to the charger. This phenomenon is also called “magnetic coupling,” because both coils are resonating (changing their magnetic fields) in a synchronized manner at the same frequency. Through this coupling, energy is wirelessly transferred from the charger’s coil to the device’s coil where it is transformed into alternating current, which is then rectified to direct current (DC), so that it can charge the battery as described earlier.
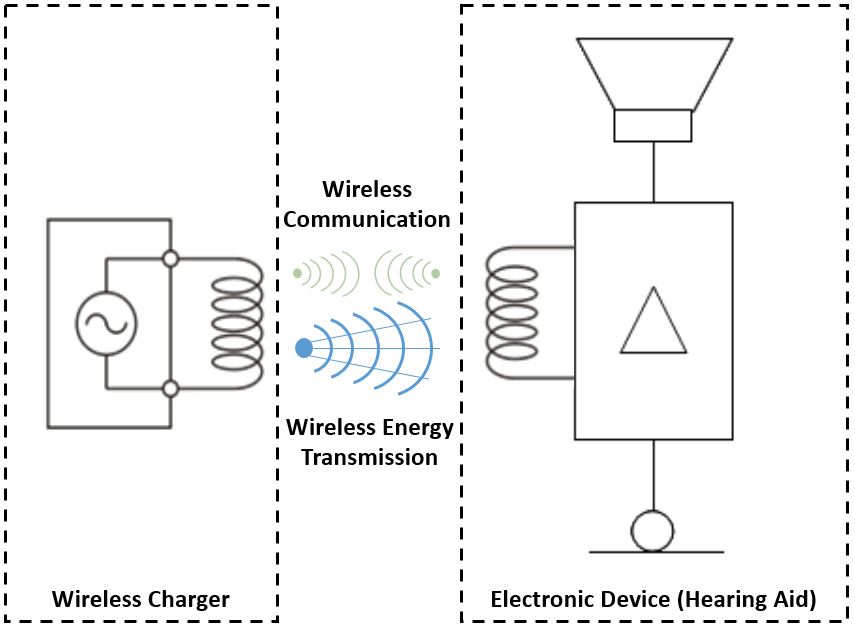
Figure 2. Wireless (also called inductive) charging. Left: The main voltage connected to the charger is converted into high frequency alternating current (AC). The alternating current flowing within the transmitter coil creates a varying magnetic field. Mid: The magnetic field variation extends to the receiver coil if both are placed within a specified distance. This process is also called inductive or resonant coupling because both coils change their magnetic fields with the same frequency. Right: The changing magnetic field generates alternating current in the receiver coil of the electronic device. This current is converted into direct current (DC) used to charge the battery.
Inductive charging has the advantage of not needing contacts, which means it allows us to design a completely sealed battery module. This offers many advantages, including increased ease of use because exact placement of the device in the charger’s cradle is not required. Another advantage is that for users, there is no hassling with opening and closing a battery door. Additionally, there are no electronic contacts that can collect dirt and debris. Improved water, sweat, and dust resistance is yet another advantage. Finally, these systems are in compliance to all country regulations that do not allow electrodes on the device (e.g., Japan).
In summary, Li-Ion batteries, in combination with inductive charging, define a new milestone in the history of environmentally friendly, robust, and easy-to-use hearing aids.
What’s Next?
Li-Ion batteries, together with inductive charging, can be regarded as a benchmark in the industry and the first step into a new world of power-efficient hearing aids. Yet this is still a young technology, and we can expect more steps forward in the near future.
- Improved capacity. New lithium-ion chemistries will come up with energy density improvement by 5 – 10 percent per year. We expect it to double today’s capacity by 2025.
- New form factors. Since Li-Ion batteries allow for a very flexible design, manufacturers might develop completely new housing concepts for hearing aids. Batteries could be more seamlessly integrated in the housing.
- Fast recharge. The next generation of lithium-ion, so-called lithium polymers, might be charged at high current in less than ten minutes without exhaustive heat production or adverse effects on the battery.
- Improved cycle life. Future batteries may show a cycle life of six years or more.
- True green technology. To make the end user more independent of supply, and to become an even more environmentally friendly technology, energy harvesting or solar chargers for hearing aids might be used in the same way they are already known for from watches and smartphones today.
Summary and Conclusion
The past ten years of hearing aid development have been characterized by significant technological progress in the field of integrated circuits and algorithm design. However, these advances have resulted in a massive increase of power consumption. That means hearing aid batteries are important considerations, not just for manufacturers but also hearing care professionals and consumers. In fact, today’s hearing aid wearers actively request environmentally friendly and hassle-free battery solutions.
Some rechargeable hearing aid battery solutions are available on the market already. An important consideration is to also offer an effective charging solution. Considering all of the pros and cons, lithium-ion technology is definitely the most promising option for truly green technology. Combined with inductive charging it is also the most user-friendly and regulation-compliant solution.
References
Abrams, H.B., & Kihm, J. (2015). An introduction to MarkeTrak IX: A new baseline for the hearing aid market. Hearing Review, 22(6), 16.
Cui, T. (2014). Can I Put a Rechargeable Battery in my Hearing Aid? AudiologyOnline, Ask the Expert 12675. Retrieved from www.audiologyonline.com
Dueber, R. (2014). What are the benefits of rechargeable batteries for rechargeable hearing aid solutions? AudiologyOnline, Ask the Expert 12966. Retrieved from www.audiologyonline.com
Kochkin, S. (2002). MarkeTrak VI: Consumers rate improvements sought in hearing instruments. Hearing Review, 9(11), 18-22.
Kraemer, B., & Naumann, F. (2016). Cellion primax: the benchmark in rechargeable hearing aid technology. Sivantos whitepaper.

